(3519) 580-155, mail@ntpf-etalon.ru
|
|
Ferrosilicon Nitride SH-SynthesisM. Kh. Ziatdinov1, I. M. Shatokhin2
1 - Tomsk State University
2 - NTPF Etalon Co., Ltd (Magnitogorsk, Russia)
In the 1970s, anew refractory known as ferrosilicon nitride came on the market; it is produced from powder with a high ferrosilicon content that is saturated with nitrogen in a high-temperature resistance furnace. The ferroalloy nitrided in this way consists mainly (70-80 %) of silicon nitride Si3N4, together with iron and/or its silicides and impurities typical of standard ferrosilicon with 75 % Si. ferrosilicon nitride is intended for use as a strengthener in unshaped refractory mixtures. Tap-hole and gutter materials for blast-furnace production made with the addition of ferrosilicon nitride prove highly effective and are widely used, especially in Japan. The ferrosilicon nitride content in unshaped refractories may vary widely (5-30 %), depending on the operating conditions. In particular, the size of the blast furnace and the conditions of hot-metal and slag discharge have a considerable influence on the composition of the tap-hole mud and channel materials. In all circumstances, however, the introduction of nitrided ferrosilicon increases the strength and wear resistance of the refractories at high temperature, increases their thermal stability and resistance to oxidation, and reduces the thermal-expansion coefficient, with simultaneous increase in thermal conductivity. It is also important that the refractories are more resistant to abrasion by the hot metal and the slag. In addition, the refractories do not shrink on repeated heating. Tap-hole mud with ferrosilicon nitride is most effective in closing the tap holes of furnaces with a large working space. Somewhat later, ferrosilicon nitride began to be used for alloying in smelting corrosion-resistant steel with an elevated nitrogen content by electroslag remelting under pressure. Thanks to the high nitrogen content in the ferrosilicon nitride used for alloying (25-30 % N) and its relatively low carbon content, it is possible to smelt high-strength steel with the maximum quantity of nitrogen (more than 1.0 %). The consumption of alloying material is minimal here, with relatively high nitrogen assimilation by the melt. In comparison with the traditional manganese and chromium alloys used for alloying, the consumption of the ferrosilicon nitride is considerably less. Despite the extensive positive experience with ferrosilicon nitride around the world, it is a new material for Russian plants. It has only begun to be introduced in Russia in the last few years, since the introduction of an industrial system for the manufacture of products from refractory inorganic compounds at NTPF Etalon Co., Ltd. (Magnitogorsk). Relatively large-scale production (in amounts of a few tons) based on self-propagating high-temperature synthesis was introduced. In self-propagating high-temperature synthesis, besides such benefits as the absence of power consumption, the speed of the process at high pressure and maximum temperature, and the simplicity of the equipment, a new approach to the selection of the raw materials for synthesis is adopted here. Instead of the expensive pure-metal powders and scarce chemical reagents typical of self-propagating high-temperature synthesis, the new method employs ferroalloys, reducing agents, and other less expensive metallurgical products. For the first time, as a result, mass production based on self-propagating high-temperature synthesis is very economically efficient. Note that, in comparison with conventional furnace technologies, self-propagating high-temperature synthesis yields higher-quality products, with a new combination of operating properties. For example, the use of vanadium-iron alloy, with a specified ratio of the components, has permitted the creation of a new alloy-fused ferrovanadium nitride of high density (-6.5 g/cm3), with the maximum nitrogen content (10-12 %). It is impossible to obtain ingots of this material (of composition Fe-VN) by traditional furnace methods. The use of ferrovanadium nitride produced by self-propagating high-teraperature synthesis ensures practically complete assimilation of ihe nitrogen by the melt and guarantees a narrow concentration range of nitrogen and vanadium within the steel. The operational properties of the new materials are improved on account of the combination of high pressure of the reacting gas and high temperature of the process. Thus, in producing ferrosilicon nitride by self-propagating high-temperature synthesis, the temperature in the combustion zone is -2000 °C, while the pressure in the working space of the reactor is 10 MPa. The introduction of ferrosilicon nitride produced by self-propagating high-temperature synthesis in Russia began with its successful use as an alloying additive in smelting transformer steel with nitride inhibition. In the converter shop at Magnitogorsk Steel Works (MMK), its use instead of nitrogen-bearing ferrochrome permitted practically 100 % increase in the nitrogen content of the steel. At NTPF Etalon Co., Ltd, a special combined method for alloying with nitrogen was developed for the production of nitrogen-bearing transformer steel. In the new method, the melt is preliminarily saturated with nitrogen, in the form of ferrosilicon-nitride pieces, in the ladle, in the production of steel with around 0.006% N. Finally, the nitrogen content is corrected by means of ferrosilicon nitride, which is preliminarily ground to powder, packed in a wire, and introduced during ladle treatment to obtain metal with 0.009-0.012 % N. Furnace ferrosilicon nitride produced abroad usually contains around 30 % N. The same material is used for alloying steel with nitrogen and for strengthening unshaped refractories. Ferrosilicon nitride produced by self-propagating high-temperature synthesis (SHS ferrosilicon nitride), which is intended for the alloying of steel, contains less nitrogen: 18-23 %. Conversion to powder wire, whose filler contains less nitrogen, increases the assimilation of nitrogen and stabilizes the smelting of metal with narrow specified limits of nitrogen concentration. In addition, SHS ferrosilicon nitride compositions of higher density and strength have been specially developed for use in chunk form in alloying in the ladle. The density of such chunks is 1.5-2.0 times that of the traditional furnace product. The strength of the chunks is several times greater, and therefore dust formation may be almost completely eliminated when using the new material, with maximum increase in nitrogen assimilation by the steel. Note that a simple technology has been developed for chunk alloying in the ladle; this technology not only ensures high and stable nitrogen assimilation in the steel but also permits an additional 5-10 % reduction in the total silicon consumption, on account of more complete silicon assimilation from ordinary ferrosilicon. In contrast to the SHS ferrosilicon nitride used to alloy steel, the nitrogen concentration is higher in compositions used as additives in unshaped refractories. NTPF Etalon Cp., Ltd has developed ferrosilicon nitride with an increased nitrogen content and reduced iron concentration. A higher silicon-nitride concentration is ensured both by appropriate selection of the raw material and by choosing conditions that ensure a product with the maximum nitrogen content. Investigation of tap-hole and channel refractories containing SHS ferrosilicon nitride shows that the ferrosilicon nitride content must be at least 75 %. After industrial tests in 2006, the MMK blast-furnace shop has now completely converted to water-free refractories containing SHS ferrosilicon nitride. The introduction of the new refractories is associated with more prolonged hot-metal discharge and a greater discharge of smelting products. In addition, working conditions for the blast-furnace staff are much improved. Note that the properties of tap-hole refractories with SHS ferrosilicon nitride are better not only than those of traditional nitride-free refractories but those of materials containing ordinary foreign furnace ferrosilicon nitride. The SHS ferrosilicon nitride is based on silicon nitride, which is a source of nitrogen when using the material as an alloying additive. If ferrosilicon nitride is introduced in refractories, the Si3N4 has a strengthening effect. Pure silicon nitride contains almost 40 % nitrogen (the stoichiometric content is 39.94 % N). It is practically inhomogeneous. At normal pressure (0.1 MPa), silicon nitride breaks down without melting at ~1900 °C. Raising the pressure increases the temperature stability of Si3N4. On contact with steel melt, the silicon nitride actively reacts, releasing nitrogen. The formation of silicon nitride is accompanied by the liberation of large quantities of heat 3Si + 2N2 — Si3N4 + 75.18 kJ/mol.
Because the reaction is highly exothermal, the synthesis of silicon nitride is possible in self-sustaining combustion. The theoretical adiabatic temperature of silicon combustion In nitrogen is exceptionally high-more than 4000 °C. However, this corresponds to the assumptions that all the silicon is converted to nitride and that there are no heat losses to the surroundings. It is also assumed that the products of synthesis do not sublimate. In practice, these conditions are very difficult to reproduce. Therefore, the experimental maximum temperature when silicon burns in nitrogen is much less: 1900-2200 °C. The adiabatic temperature of ferrosilicon combustion in nitrogen will be lower when the theoretical combustion temperature of silicon is lower, since the thermal effect of the reaction between the alloy and nitrogen is known to be less than the exothermal effect of the pure metal. This is mainly because the ferrosilicon contains a considerable quantity of iron, which, in contrast with silicon, reacts with the nitrogen practically without heat liberation, while the nitrides formed here are thermally unstable. Another factor is that silicon and iron are bound in thermally stable silicides, whose decomposition requires considerable energy consumption. The combustion temperature is calculated from the conditions of equal enthalpies of the initial materials at the initial temperature (Tо) and the products of synthesis at the combustion temperature (Tc). Thus, all the heat liberated in Si3N4 formation is consumed in heating of the products, i.e., silicon nitride and iron μ[H(Tг) - H(To)]Si3N4 + (1 - μ)[H(Tг) - H(To)]Fe = μQ,
where Q is the thermal effect of Si3N4 formation; μ is the proportion of silicon nitride in the product; H(Tо,), H(Тc) are the enthalpies of the combustion products at Tо and Tc.
The combustion temperature calculated by this formula is relatively high for alloys with different Si content. Thus, even for ferrosilicon with 45 % Si, the theoretical combustion temperature is ~2500 °C. Hence, certain thermodynamic preconditions must be satisfied for successful self-propagating high-temperature synthesis in the ferrosilicon-nitrogen system over a broad range of initial alloy composition. In fact, experiments confirm that combustion may occur for all ferroalloys containing more than 40 % Si. In many ways, the combustion of ferrosilicon in nitrogen is similar to the combustion of metallic silicon. Thus, in nitriding, it is found that the combustion products contain considerable unreacted silicon, and consequently the combustion temperature is relatively low. Such incomplete conversion of silicon to the nitride is due to the low melting point of the silicon itself (1415 °C) and the relatively low dissociation temperature of its nitride. The conversion of silicon to the nitride in the experiments is 50-60 %. The melting point of Fe-Si alloys is even lower than for silicon. In alloys with 40-80 % Si, the liquid phase is formed above 1210 °C. Hence, processes associated with melting of the initial material in ferrosilicon combustion may be more pronounced. We know that a filtrational-combustion mechanism is responsible for the nitriding of metal powders, including silicon. In the filtrational version of self-propagating high-temperature synthesis, the gaseous reagent is supplied to the combustion zone by filtration through a porous medium formed by the metallic component. In nitriding ferrosilicon, filtration is maintained by the difference in nitrogen pressure between the reaction zone and the surroundings. The pressure in the combustion zone falls steadily as a result of nitrogen absorption by the alloy at high temperature. Nitrogen must be supplied to the combustion zone by filtration from outside because in ordinary conditions (at normal atmospheric pressure and room temperature), the quantity of nitrogen in the pores of the ferrosilicon powder is very small and clearly insufficient to maintain nitriding by self-sustaining combustion. Moreover, calculations show that the nitrogen in the pores is insufficient for nitriding of the ferrosilicon even at elevated pressure. For example, at 10 MPa, the nitrogen within the powder is only sufficient to bind 1-5 % Si in the nitride, depending on the ferrosilicon composition and the porosity of the powder batch. That degree of nitriding may increase the temperature in the Fe-Si-N2 system by no more than 200-500 °C, which not only is insufficient for a self-propagating process but also eliminates the possibility of reaction. For filtrational combustion, the process parameters depend strongly on the characteristics of the reacting system that determine the filtration conditions: specifically, the nitrogen pressure, the dispersity of the ferrosilicon powder, and the porosity and geometric dimensions of the batch. In addition, the parameters of ferrosilicon combustion in nitrogen depend on the initial conditions determining the thermal balance in the system: in particular, the quantity of inert components, the initial temperature of the ingredients, the introduction of activating additives, and the possibility of melting of the initial powders and/or reaction products. The influence of the silicon concentration in the initial ferrosilicon and the nitrogen pressure on the combustion rate, the degree of nitriding of the alloy, and the maximum temperature in the reaction wave is illustrated in Fig. 1. The results are obtained for self-propagating high-temperature synthesis in 15-1 experimental apparatus. A quartz-glass window permits visual observation of the combustion process and video recording of the combustion-wave propagation. The laboratory apparatus also includes a system for continuous measurement of the combustion temperature by means of thermocouples and computer recording of the temperature profile. Nitriding is based on FS90, FS75, FS65, and FS45 ferrosilicon powder (State Standard GOST 1415-93), with 89.9, 79.4, 68.1, and 48.25 % Si, respeclively, and 0.06, 0.08, 0.07, and 0.23 % C, as well as standard quantities of other impurities. In all cases, the particle size of the powders is no more than 0.08 mm. Fig. 1. Influence of the silicon content in ferrosilicon on the combustion rate (a), nitriding degree (b), and combustion temperature (c) when PN2 = 3 (1) and 7 MPa (2).
According to the Fe-Si phase diagram, alloys with a high Si concentration consist of two phases: silicon and iron disilicide FeSi3. With increase in Si content in the alloy, the content of free silicon also increases, as confirmed by X-ray phase analysis of the initial ferrosili-con p owder. Thus, whereas FS65 alloy contains no more than 25 % free silicon, FS90 alloy contains 80-85 % free silicon. Low-silicon FS45 also has a two-phase structure: about 70 % FeSi2, and the remainder FeSi. As expected, increase in the initial content of silicon is accompanied by more vigorous reaction of the silicon with nitrogen; this corresponds to considerable increase in combustion rate and increase in nitrogen concentration in the products. At the same time, the degree of nitriding of silicon in the alloys declines with decrease in their iron content. Thus, despite the relatively low nitrogen content in FS45 ferrosilicon, the degree of silicon conversion to the nitride is a maximum: almost 90 %, as against 65 % for the alloy with 90 % Si. The dependence of the combustion rate, nitrogen content, degree of nitriding, and combustion temperature on the silicon content in the ferrosilicon does not depend on the nitrogen pressure. Increase in the pressure strongly affects the combustion rate, but hardly changes the degree of nitriding of the alloys. With increase in pressure, the combustion temperature rises somewhat. X-ray phase analysis of the combustion products of ferrosilicon in nitrogen shows that, over the whole range of initial parameters, the basic phase is β-silicon nitride. No pronounced quantities of α-silicon nitride are seen. This is probably because the α-structure is only stable up to ~1400 °C and is converted irreversibly to the β-structure at higher temperatures. The combustion temperature of ferrosilicon in nitrogen is more than 1750 °C for all the initial conditions considered, and therefore α-Si3N4 formation is unlikely. Typical X-ray patterns of the nitriding products of FS45 and FS75 are shown in Fig. 2. 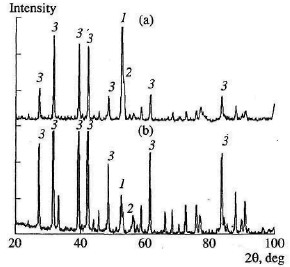 Fig. 2. Typical X-ray patterns of the nitriding products of FS45 (a) and FS75 (b) ferrosilicon:
(1) Fe; (2) FeSi; (3) - βSi3N4 In studying the nitriding of ferrovanadium in conditions of self-regulating combustion, it was found that the absorption of nitrogen in stages is possible, depending on the aggregate state of the products behind the combustion wave. If the liquid content in the combustion product is high, a gas-impermeable structure is formed immediately behind the combustion wave. When refractory components form a large proportion of the reaction products, the material remains gas-permeable, and nitrogen absorption continues beyond the combustion wave in bulk-combustion conditions. Note that a second stage to complete the combustion will not occur unless a permeable structure is retained and conversion in the combustion wave is incomplete. The latter condition generally holds in practice, since the powder particles are too large to permit combustion in kinetic conditions, i.e., conditions eliminating diffusional and filtrational obstacles to chemical reaction. Analysis of the sample structure shows that, in all cases, considerable permeability of the ferrosilicon nitride persists. The porosity of the combustion products is 35-55 %, depending on the conditions of synthesis. With such high porosity behind the combustion front, intense absorption of nitrogen is possible. To determine the contribution of such nitriding to the total nitrogen content in the combustion products, combustion is interrupted by sharply reducing the pressure in the working volume of the reactor, and then introducing argon. Chemical analysis of the samples quenched in this way shows that the final combustion stage considerably increases the nitrogen content. The proportion of nitrogen absorbed after passage of the laminar-combustion wave as a result of the final combustion stage may amount to 30 %. The great contribution of the final nitriding stage to the total nitrogen content may be attributed, in particular, to the weak pressure dependence of its content in the product. The typical structure of ferrosilicon nitrided by combustion is shown in Fig. 3.  Fig. 3. Macrostructure of SHS-ferrosilicon nitride
Exceptionally uniform distribution of nitrogen over the volume of the product is important here. In fact, ferrosilicon nitride is a composite consisting primarily of silicon nitride. The binder is iron and its silicides. With a high nitrogen content in the product, the proportion of free iron rises; with decrease in the degree of nitriding, the quantity of silicides increases. Since the density of Si3N4 (3.19 g/cm3) is much less than that of iron and its silicides, the content of silicon nitride in the product is very high (80-95 vol %). A 0.15 m3 SHS reactor has been developed at NTPF Etalon Co., Ltd for the industrial production of SHS ferrosilicon nitride and other materials. It takes the form of a thin-walled metal vessel with systems for cooling, gas injection and extraction, ignition, and sealing. Thanks to a reactor design with an accelerated-cooling system, SHS technology went into operation on an industrial scale; the working temperature is up to 2200 °C, with one-lime loading of up to 0.3 t of batch. The new sealing systems permits safe and reliable synthesis in filtrational combustion ai a nitrogen pressure up to 15 MPa. The time required to open and seal the equipment is reduced to a minimum. The shop for the production of ferrosilicon nitride and other SHS materials includes sections for crushing and fine grinding, drying and charging of the crucibles, and synthesis, as well as dispatcher and central control panels, a certified laboratory for intake monitoring of the raw materials, and a store for the initial materials and the final products. In the synthesis section, there are 20 SHS reactors; the total area of the shop is 3000 m2. The new shop can produce up to 10 t of various materials per day. The universal design of the SHS reactors permits the production of a wide range of materials based on inorganic refractory compounds of nitrides, borides, carbides, sulfides, and soon. Synthesis in reactive gas (nitrogen), argon, or vacuum is possible. The table presents the compositions of the ferrosilicon nitride produced and supplied by NTPF Etalon Co., Ltd. The products FSN35 and FSN30 are intended for the refractory industry. JSC Spetsremstroi (Magnitogorsk) uses such ferrosilicon nitride to produce water-free tap-hole material of type SiO2 – Al2O3 - SiC - С. Mainly FSN20 and FSN15 are used for alloying; they ensure high and stable assimilation of nitrogen by the steel. Thus, SHS technology for the production of a new material, ferrosilicon nitride, has been developed and industrially introduced by NTPF Etalon Co., Ltd, and the first Russian system for the large-scale (in amounts of a few tons) production of metallurgical materials based on refractory inorganic compounds has been created. Published in "Steel In Translation" Volume 38, Number 1, January 2008 (article in pdf)
Ferrosilicon nitrideNITRO-FESIL®, produced by NTPF Etalon Co., Ltd: Other publications about SHS |
